
Almofada de cunha para pernas – rampa triangular de espuma viscoelástica projetada para reduzir o refluxo, ronco – Suporte médico de elevação de perna tamanho king para pós-cirurgia – Gravidez – Liberador
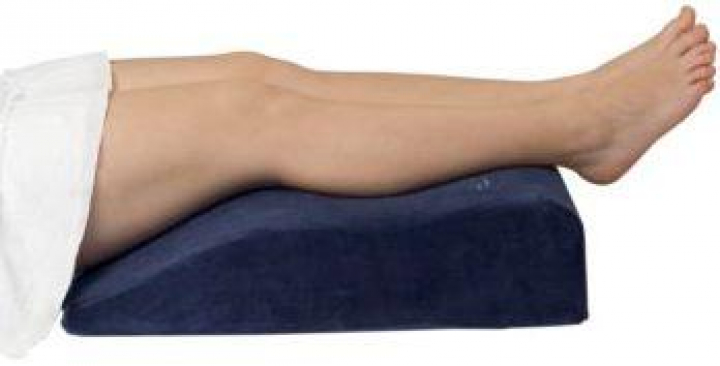
Almofada de posicionamento em cunha para pernas em visco-elástico com capa impermeável 65x45x14cm 2401931 :: Servisfarma

Almofada de posicionamento em cunha para pernas em visco-elástico com capa impermeável 66x70x30cm Biopruf GT140006 :: Servisfarma

Buy Almofada de elevação da perna com tampa de espuma de memória gel de resfriamento, travesseiro de cunha para resolver as costas & amp; Dor nas pernas, refluxo ácido, azia, ronco, gravidez

Travesseiro de elevação da perna portátil apoio inflável Suporte rampa almofada perna perna alívio dor | Fruugo PT

Almofada inflável do reforço para os joelhos das pernas mais baixa volta cunha almofadas recuperação pós operatório perna circulação de sangue promover o apoio|Travesseiros| - AliExpress

Travesseiro Para Descanso De Perna,Almofada De Descanso Com Almofada Para Perna,Gel Respirável E Lavável,Travesseiro De Cunha Com Memória De Elevação - Buy Leg Rest Pillow,Leg Elevation Pillow,Leg Wedge Pillows Product on Alibaba.com

Almofada Em Cunha Inflável-Leve E Portátil Para Dormir , Leitura , Pós-Cirurgia Elevação Das Pernas-Triangular Com Capa Lavável Melhor - Desconto no Preço

Desconto Venda Quente Portátil Inflável De Elevação Em Forma De Cunha Perna Travesseiro Para Dormir, Suporte De Joelho Almofada Com Bomba De Ar Entre A Perna > Casa & jardim - Patatine.pt














